Guinea worm disease is a debilitating infection of humans caused by the parasitic nematode Dracunculus medinensis. Historically, Guinea worm disease has afflicted 3.5 million people per year across Asia and Africa, but global eradication efforts since the 1980s have reduced the number of human cases to only 28 in 2018 – see here for the most up to date reports.
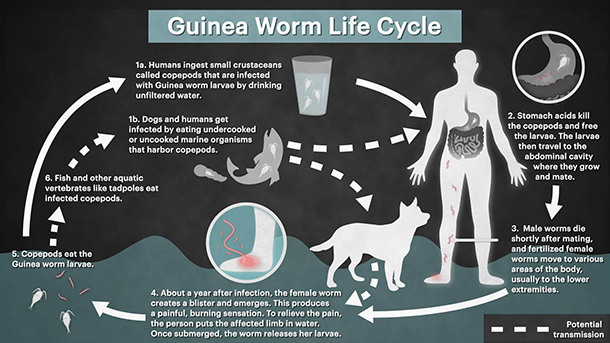
To really appreciate the desire to eradicate this disease we first need a description of the parasites gruesome life cycle. The transmission cycle of Guinea worm in humans starts after a female worm emerges from its host and releases larvae into a water source, where an intermediate host (copepods) then ingests the larvae. The classic transmission pathway to humans is through the consumption of drinking water containing infected copepods. However, a novel pathway has recently been hypothesised, and transmission is now thought possible through the ingestion of raw fish or frogs that have themselves eaten an infected copepod. Once consumed by the host, larvae migrate through the digestive tract and into the host’s connective tissues, where they mature and mate. Fertilized females then lie dormant for around a year, growing up to 1 m in length. After this incubation period, the female worm creates a painful blister on its host from which it emerges once contact with water is made, and the cycle starts again.
If the eradication program is successful, Guinea worm will be the second human disease in history to be eradicated (after smallpox). However, the closing stages of the global eradication campaign may be affected by the recent detection of clusters of infection among dogs in Chad (all along the Chari River) and in Ethiopia (restricted to a handful of rural villages in the West of the country). The large number of dog infections in Chad suggests that dogs are capable of maintaining a reservoir of disease in the environment that could result in human cases. It is therefore important that the eradication campaign interrupts transmission to both dogs and humans. However, while the transmission pathways in humans are fairly well understood there is uncertainty around how dogs acquire the infection. What’s more, educating communities on how to filter water and the provision of clean water was a key method for reducing human cases, but these methods are not as easily applied to free-ranging animals. A better understanding of dog ecology in relation to Guinea worm transmission is required to help identify the key transmission pathways for dog infections, and to better guide the eradication program on appropriate intervention strategies.
In 2016 I joined a team at Exeter University to conduct a pilot investigation into the ecology of free-ranging domestic dogs in rural Chad in relation to Guinea worm infection. Using the apriori hypotheses from the classical and novel transmission pathways, we set out to understand correlates for the risk of infection, with a particular focus on dog diet and dog activity around water bodies. To achieve this we interviewed dog owners, used GPS units to track the movements of dogs over 2 weeks, and took whisker samples for dietary isotope analysis. We found some evidence for the classical transmission pathway, whereby dogs living in households that provided water to their animals had a lower risk of having had Guinea worm. We also found evidence for the novel transmission pathway, and dogs that ate more fish were more likely to have had a history of infection.
We returned to Chad in 2018 to conduct a follow up study, with a larger sample size of dogs followed over a longer duration (3 field seasons, each 2 months long). The results of this second investigation showed that the amount of fish in the dogs’ diet was again reflective of the risk of infection, providing good evidence for the novel transmission pathway for dog infections in Chad. We were unable to reproduce the effect of water provision, and found little evidence to suggest more activity by dogs around water bodies was associated with an increased risk of infection. This is not to say the classical transmission pathway should be ruled out as (1) our study relied on dog owners reporting water provision which may miss important variation (e.g. seasonal differences in water provision and usage by dogs), and (2) our methods looked at the general activity of dogs around water bodies, but it is likely that the risk of infection is better captured by activity around spatially discrete water bodies known to be infected. Eradication efforts may therefore benefit from studies specifically focused on dog husbandry, the drinking habits of dogs, more advanced methods of identifying water bodies (from satellite imagery) and studies that develop tools for detecting the parasite in water bodies (a tool that is in development).
In Ethiopia, we conducted a pilot study similar to that in Chad. This small scale investigation revealed that, in contrast to Chad, the classical transmission pathway may be more important. The dogs in Ethiopia had little to no fish in their diets, and no link could be made between diet and the history of infection. However, dogs with owners that reported a higher rate of water provision were more likely to have had a history of infection. This is counterintuitive, but may be explained by the education provided by the eradication program. Households with a history of dog infections are likely to have had more exposure to the eradication programs education efforts, and would therefore be more aware of methods to prevent dog infections. Despite this, our results point more towards the classical transmission pathway, but a follow up study would be beneficial to further our understanding of dog infections in Ethiopia.
Our research has provided some valuable insights into dog infections, but there is still a considerable amount of research required to help the eradication program break through and conquer Guinea worm. I believe that a ‘One Health’ approach is greatly needed, and surveillance efforts need to combine the surveillance of humans and dogs with that of the environment (water bodies), while also considering factors that will be important to the lifecycle of the parasite e.g. dog ecology, dog husbandry and hydrology.









